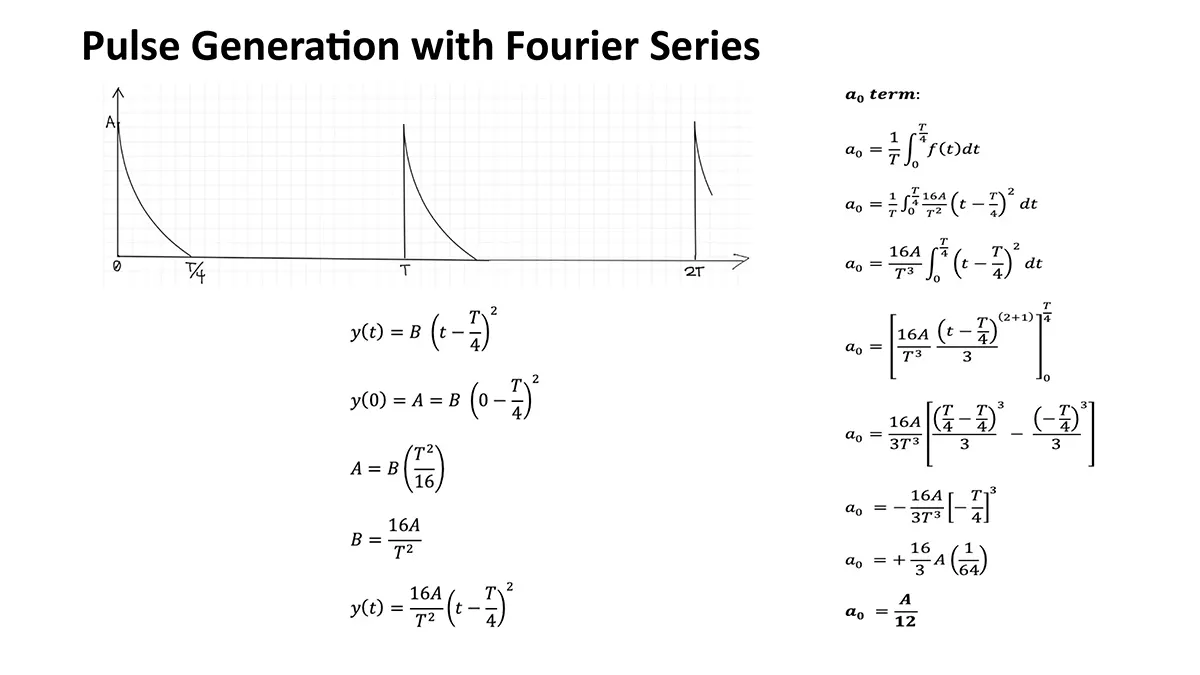
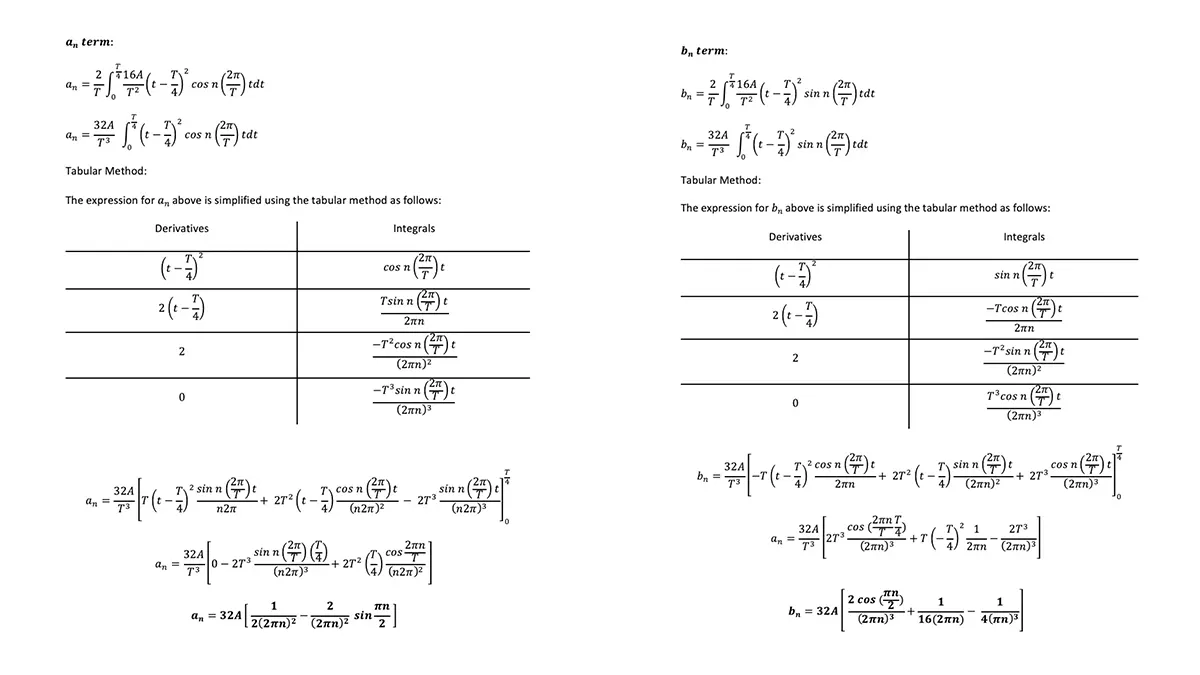
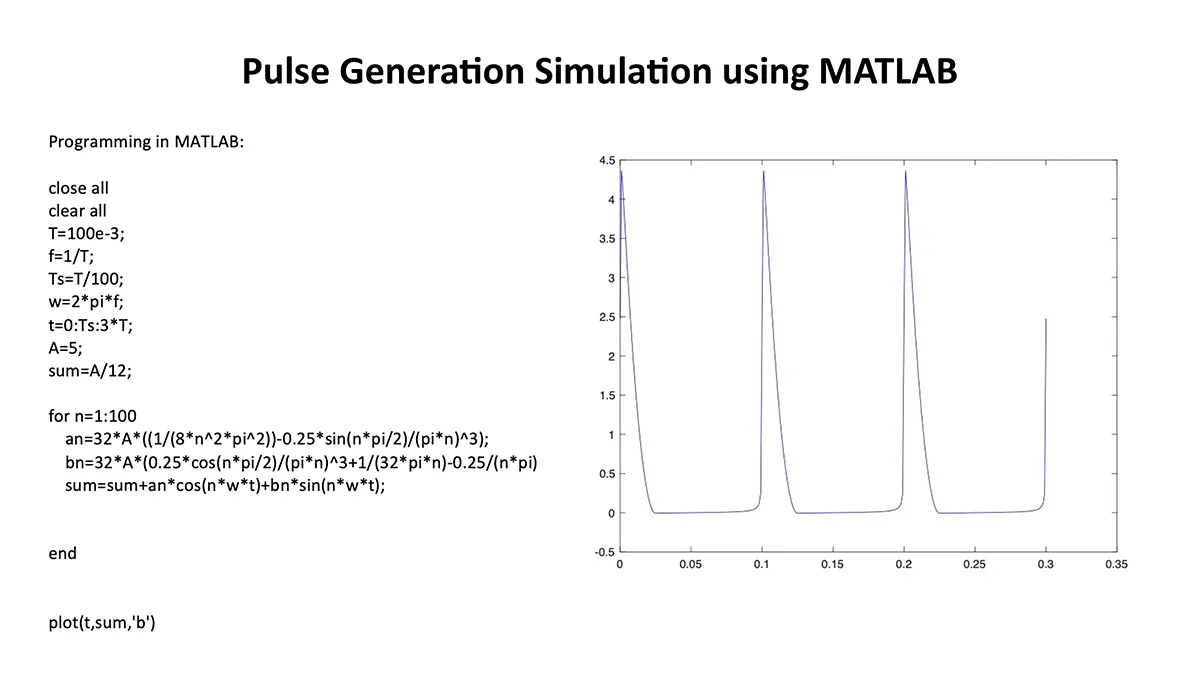
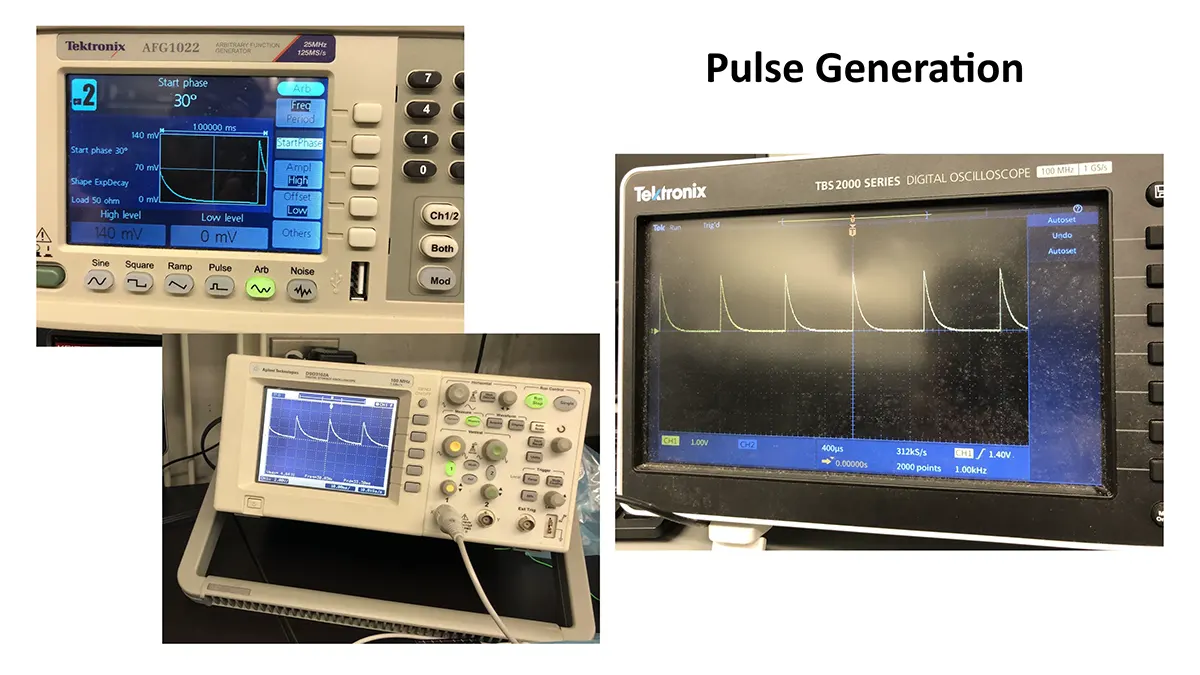
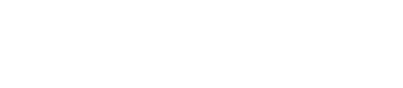Merkel cell carcinoma (MCC) is a rare but aggressive cutaneous malignancy. At least one-third of patients experience local or distant recurrence and the 5-year survival rate is approximately 14% in patients presenting with distant metastases. MCC tends to occur in sun-damaged skin in the elderly population, with a male to female ratio of 8:1. Various mechanisms drive MCC development: (1) integrated Merkel cell polyoma virus (MCPyV) in approximately 80% of cases or (2) high ultraviolet light–induced somatic mutational burden specific to MCPyV-negative MCCs in US. There is controversy surrounding the cell of origin of MCC but it has been postulated that possible cells of origin include dermal pluripotent stem cells or neural crest cells. Neural crest derived tissues express acetylcholine receptors (AChRs) (PMID: 7649378). These receptors are critical for migration of neural crest derived cells to their corresponding tissues during development. In fact, recent reports demonstrate neural crest derived melanoma and numerous non-Merkel cell neuroendocrine tumors express AchRs. AchRs have been linked to cell proliferation and cell migration and may serve as potential therapeutic targets in MCC.
Identifying Therapeutic Strategies in Merkel Cell Carcinoma
Understanding the cholinergic anti-inflammatory response in the context of SARS CoV-2: Uncovering a potential therapeutic strategy to counteract the cytokine storm in COVID-19
One of the most significant complications of Coronavirus disease 2019 (COVID-19) is the dysregulated immune response, leading to excessive inflammation and cytokine storm. This chronic inflammation contributes to the severity of the disease and the poor outcomes observed in many patients. To address this issue, this one year proposal aims to investigate the role of the alpha7 nicotinic acetylcholine receptor (α7-nAChR) and the cholinergic anti-inflammatory pathway (CAR) in the regulation of inflammation in COVID-19 patients. Our preliminary findings show that the spike protein of the COVID-19 causative agent, SARS CoV-2, reduces the levels of the α7-nAChR ion channel in macrophages and neuronal cells. This is significant because activation of α7-nAChR reduces inflammation by preventing macrophages from producing inflammatory cytokines. The main objective of the study is to investigate the role of the α7-nAChR in the inflammation/cytokine storm and neurological manifestations present in patients suffering from COVID-19. Our central hypothesis is that SARS-Cov 2 infection impairs CAR function in human MDMs, facilitating the development and progression of a chronic inflammation/cytokine storm in COVID-19 patients. To that end, we propose the following three objectives: Aim 1 seeks to determine whether the Severe Acute Respiratory Syndrome coronavirus 2 (SARS-Cov 2) spike protein can disrupt the CAR in human monocyte-derived macrophages (MDMs), leading to chronic inflammation and cytokine storm. Aim 2 investigates whether nicotine pre-exposure can prevent the downregulation of α7-nAChR expression in MDMs and neuronal cells exposed to SARS-Cov 2 spike protein, potentially offering a preventive and therapeutic strategy for COVID-19 patients. Finally, Aim 3 aims to identify effective smoking cessation medication (SCM) pharmacological strategies to counteract inflammation in COVID-19 patients by activating the CAR in MDMs.
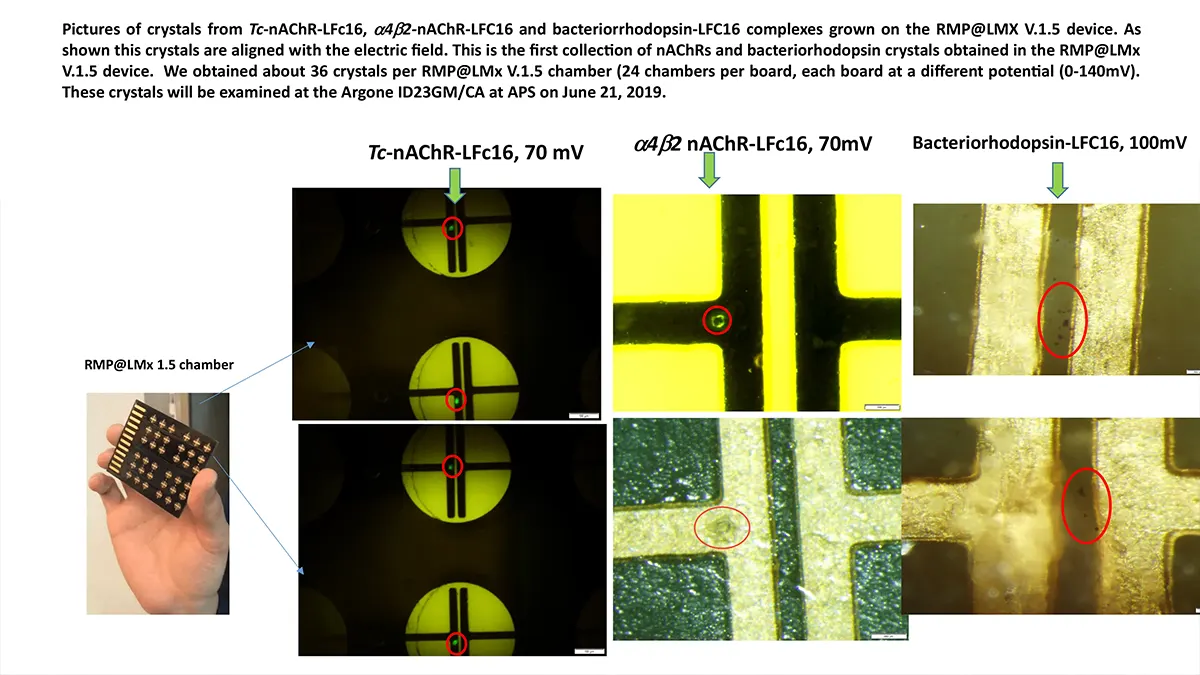
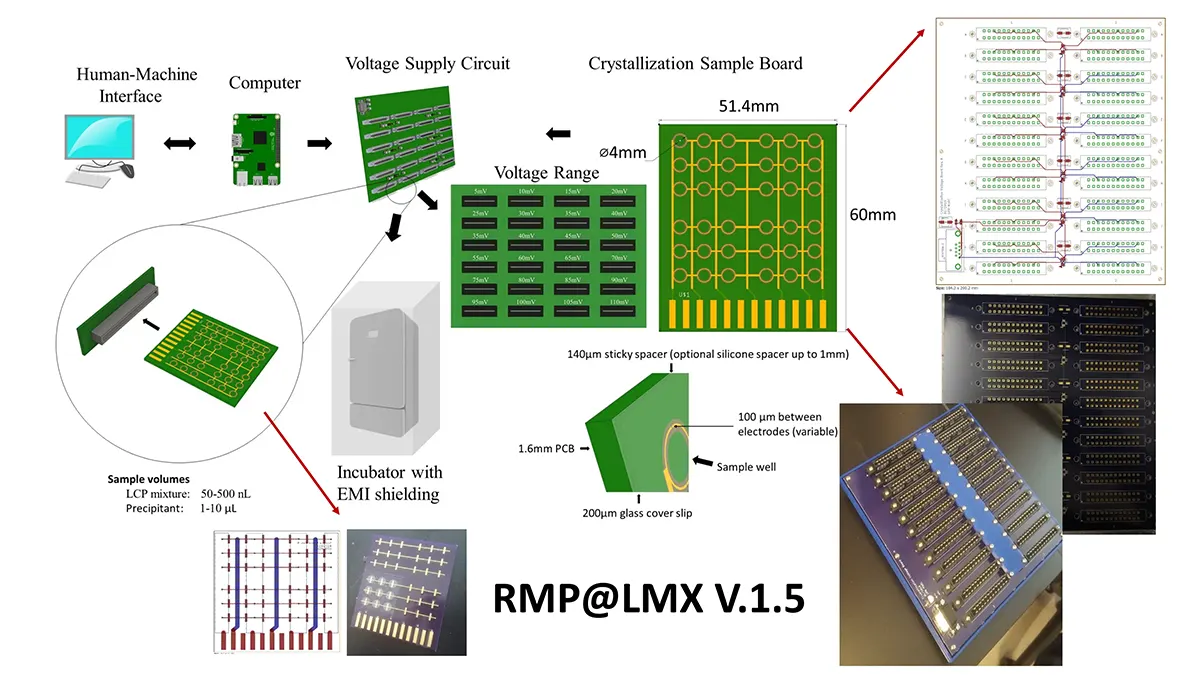
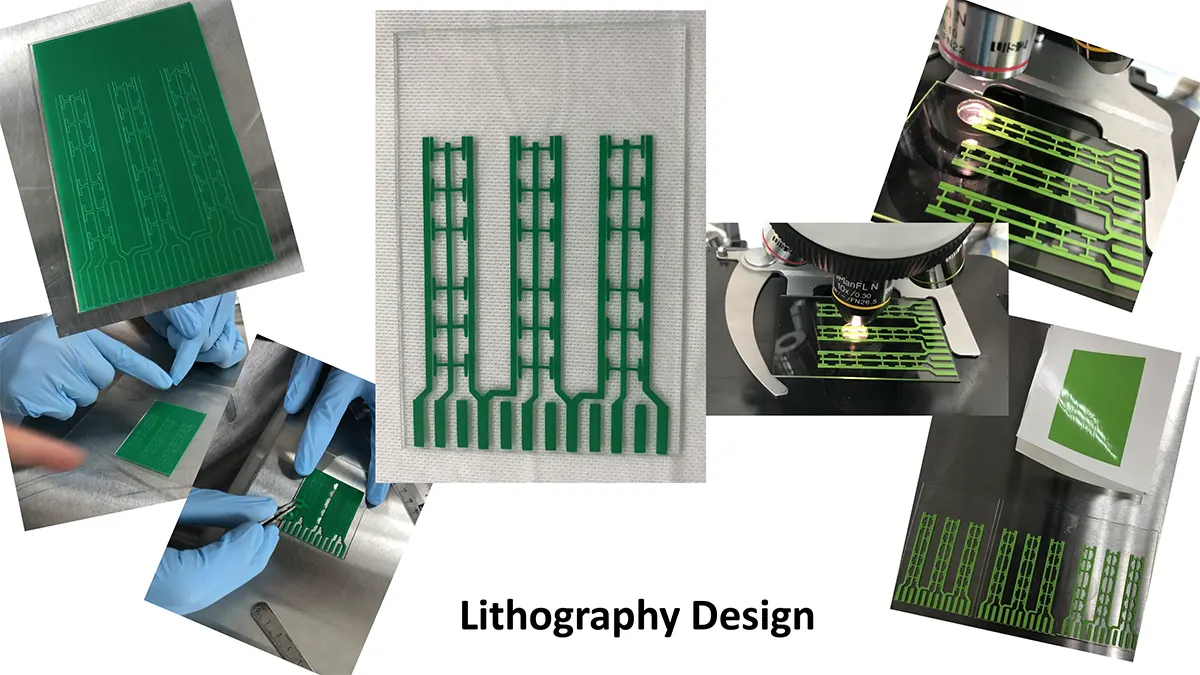
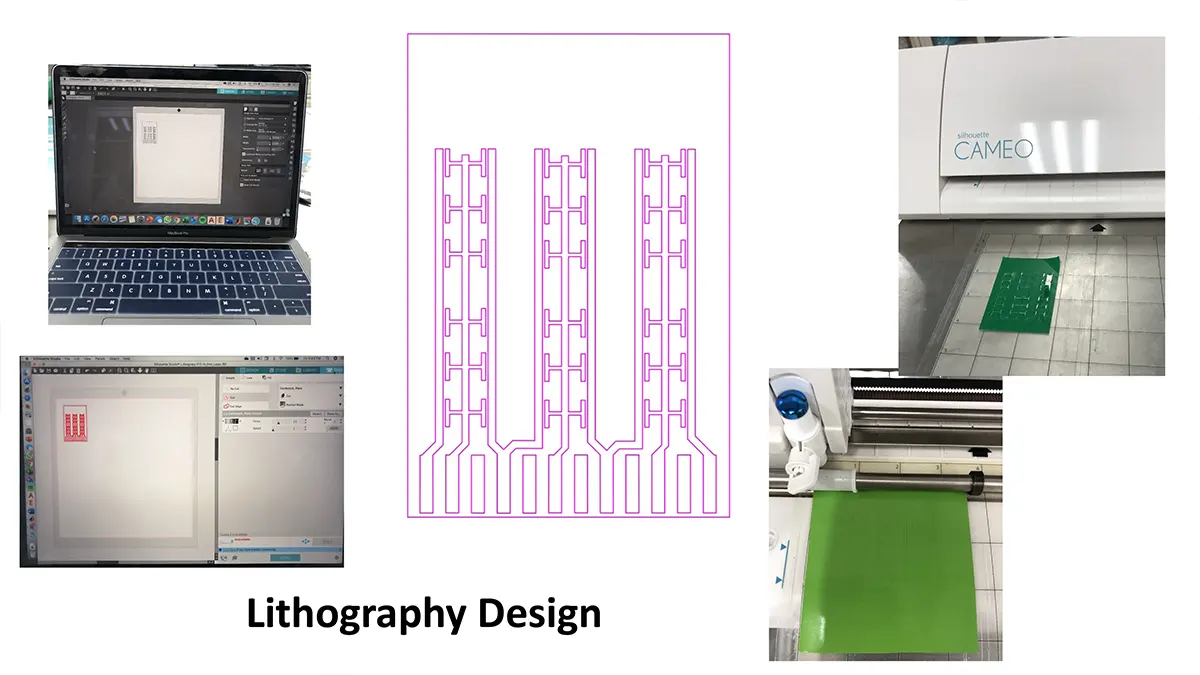
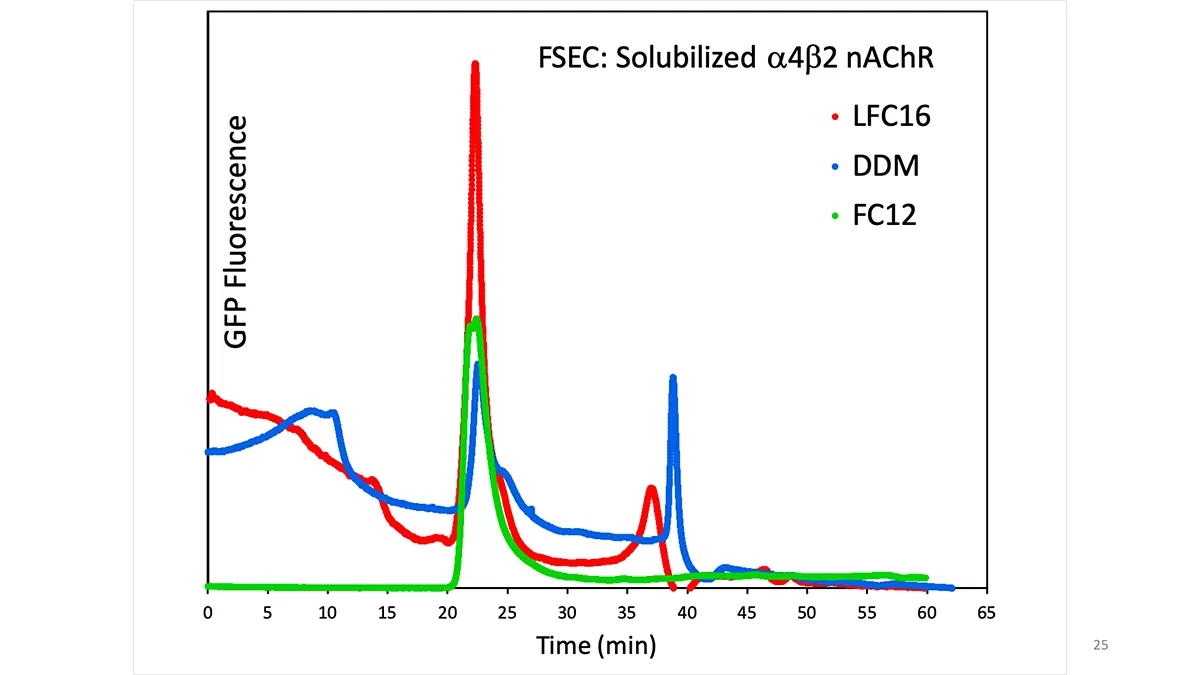
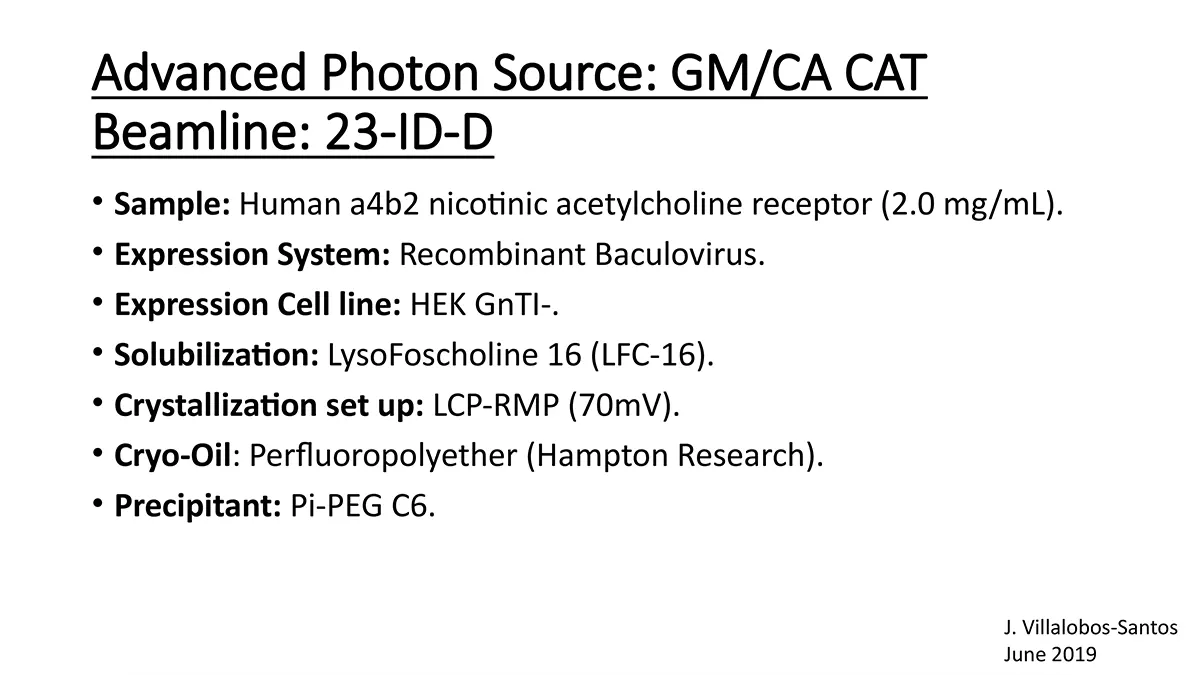
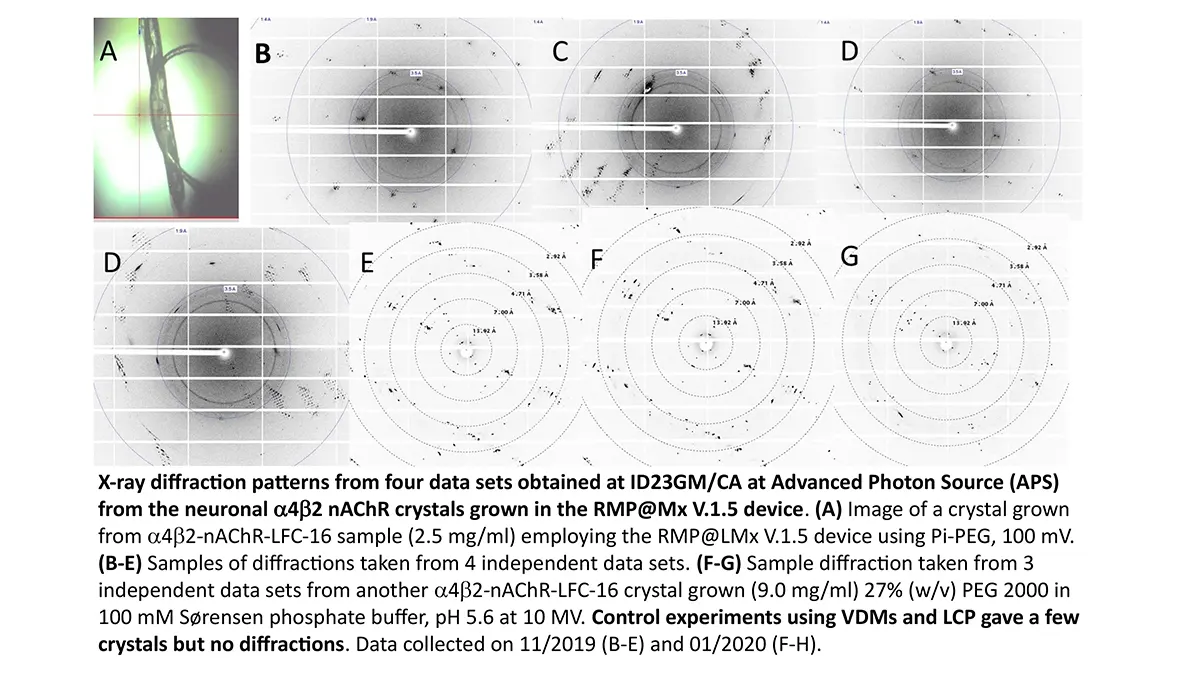
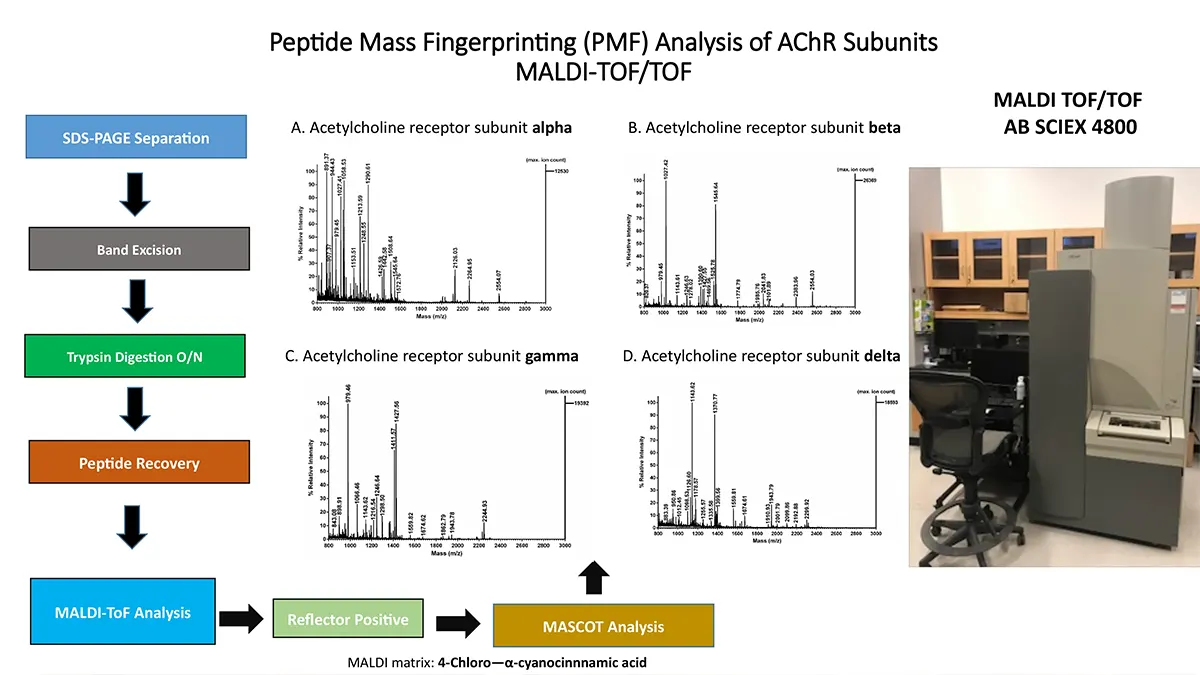
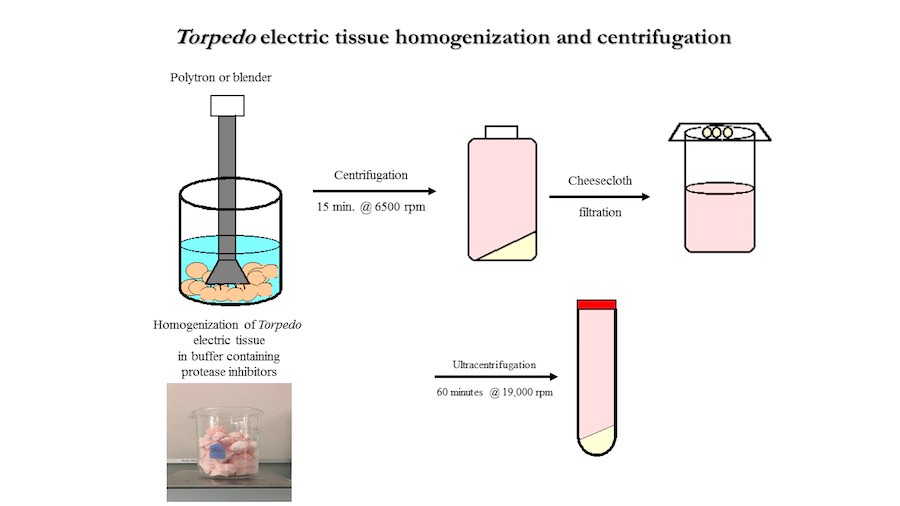
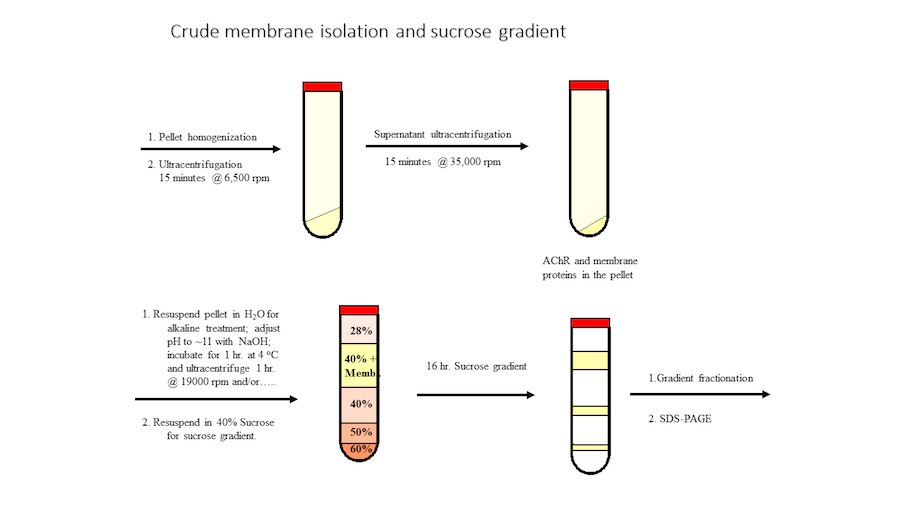
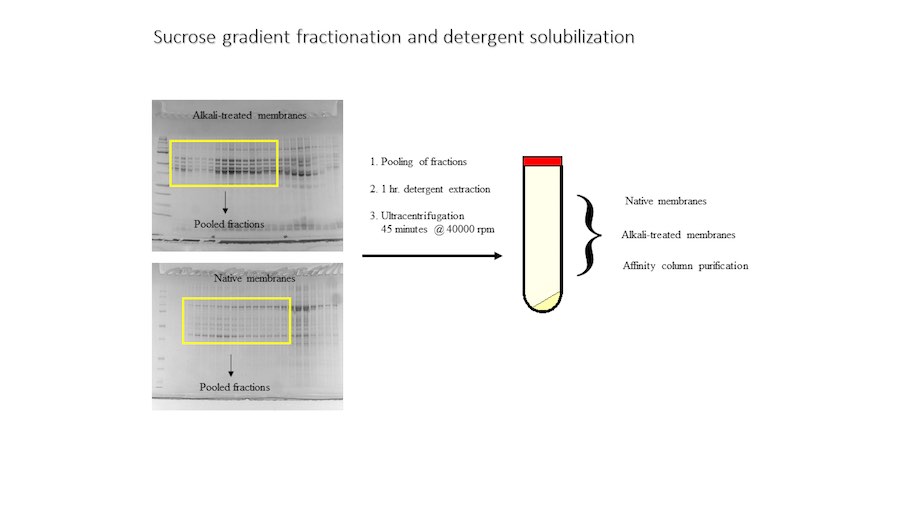
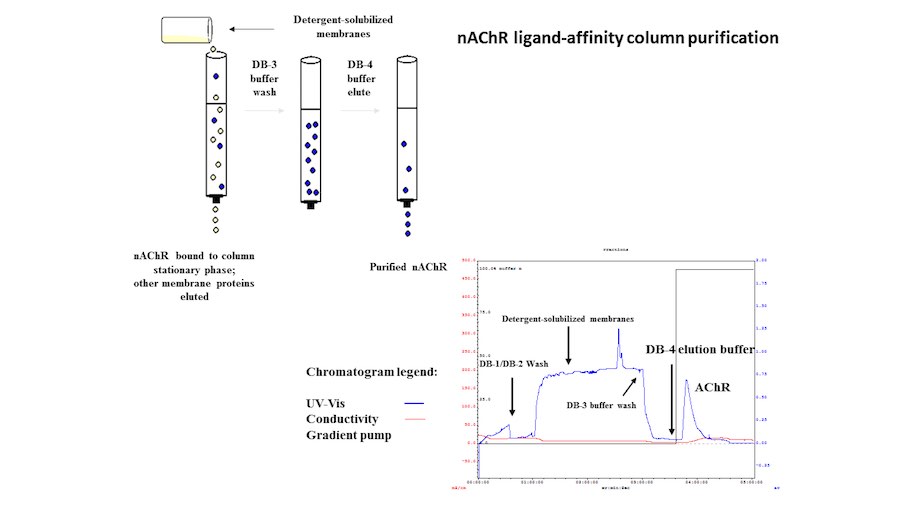
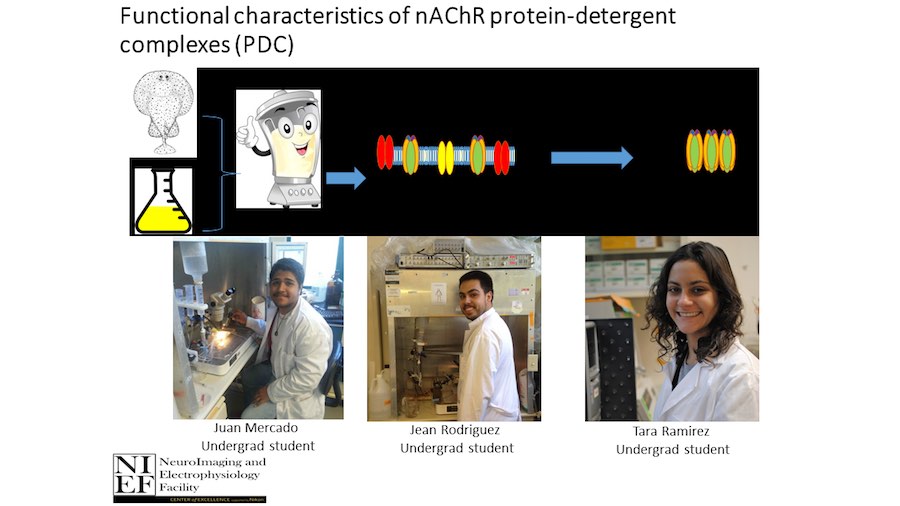
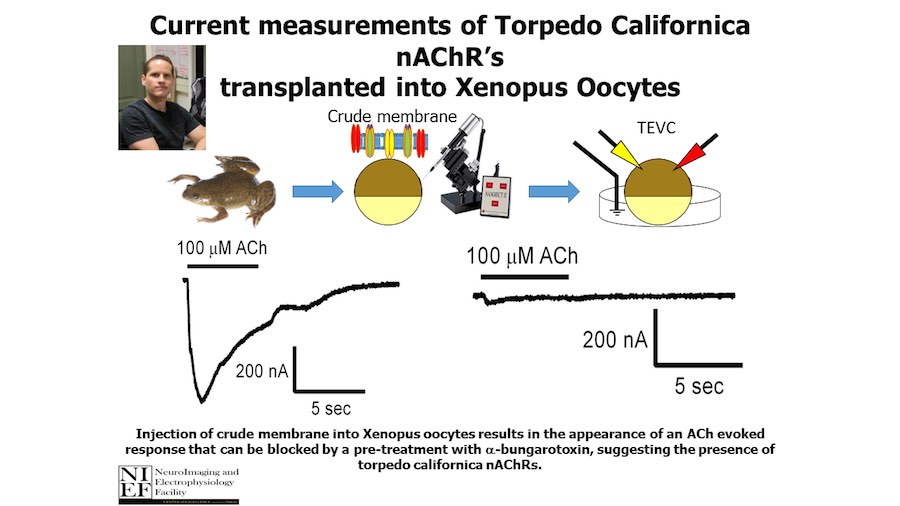
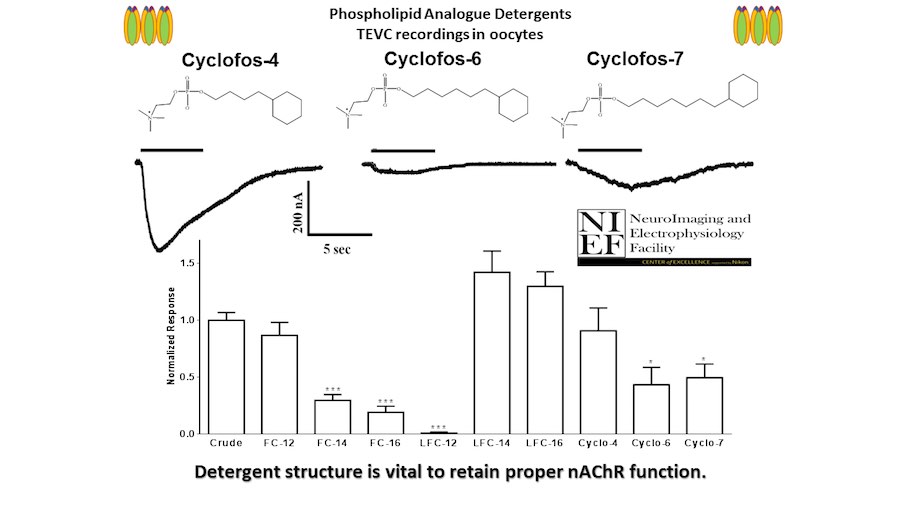
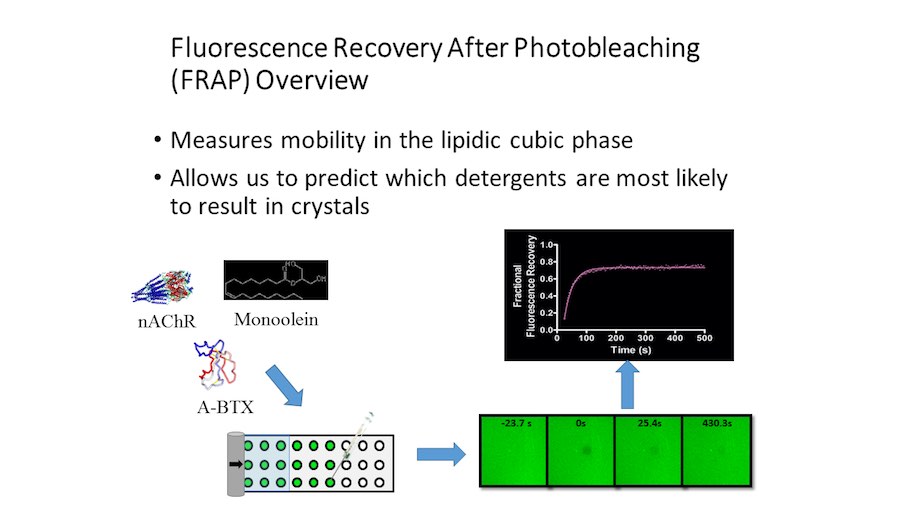
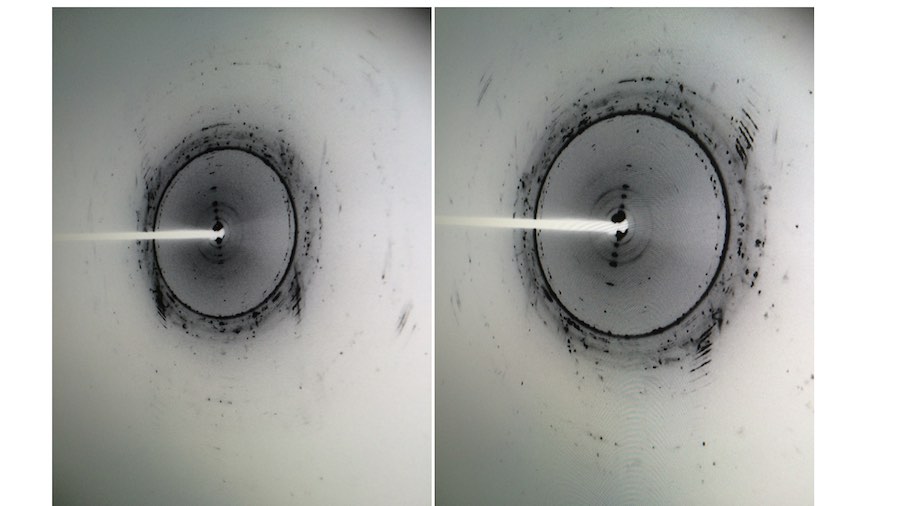
Crystallization of nicotinic acetylcholine receptors at Resting Membrane Potentials (RMP)
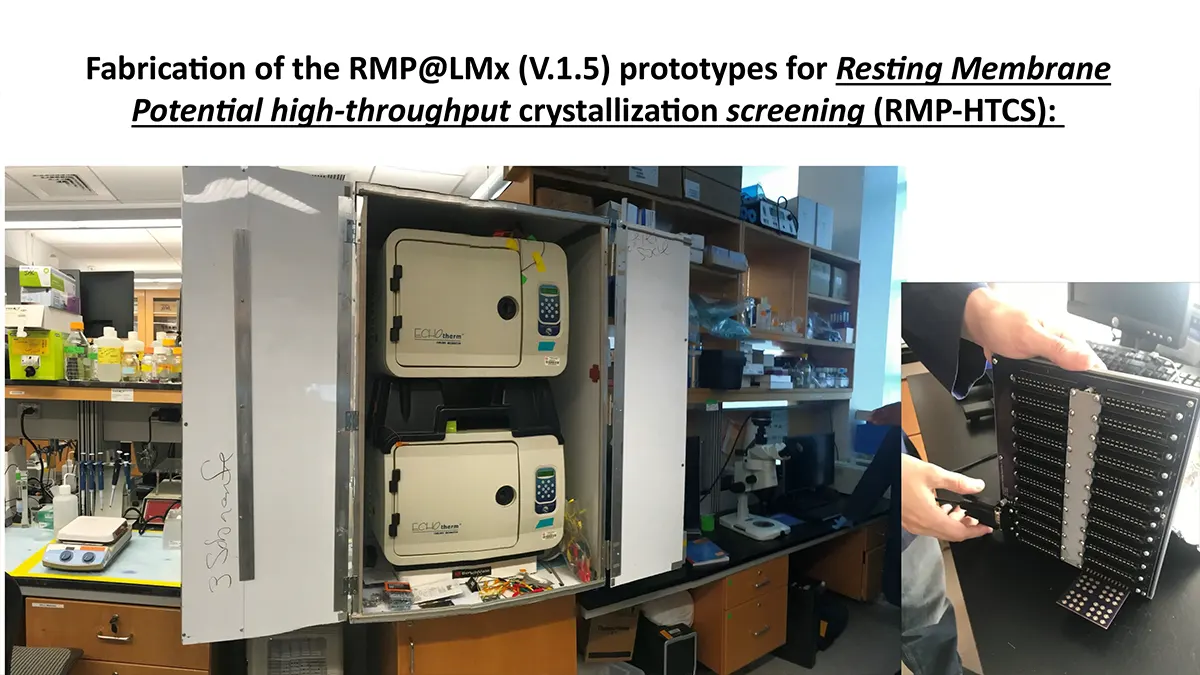
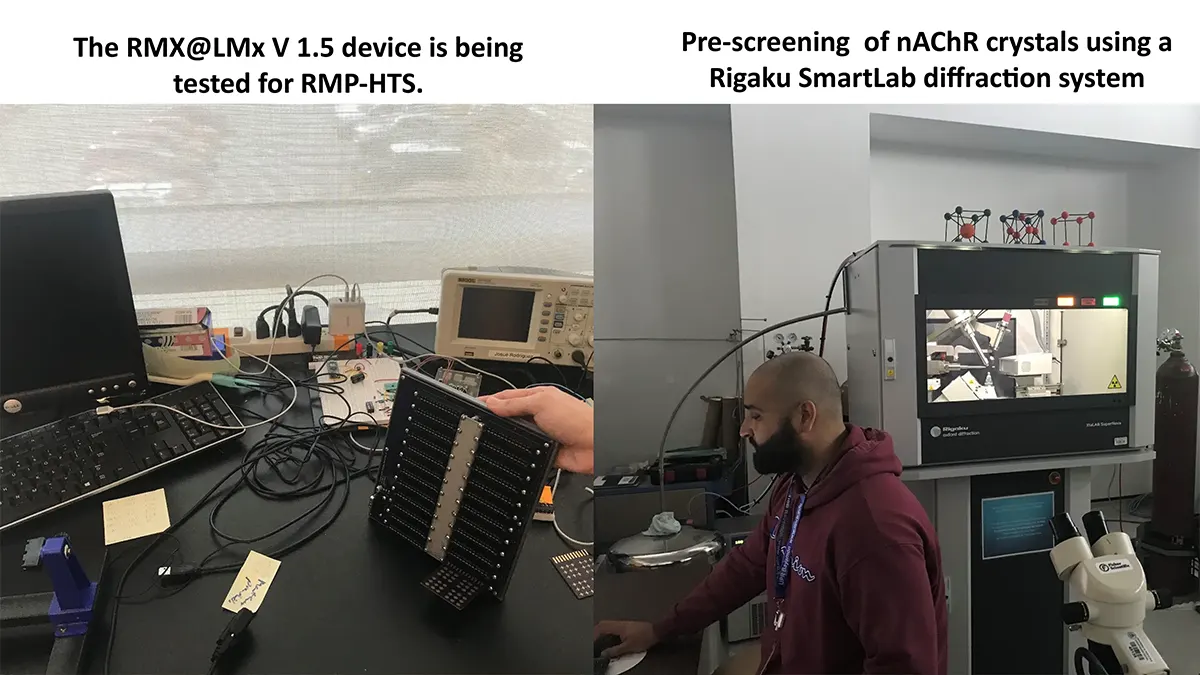
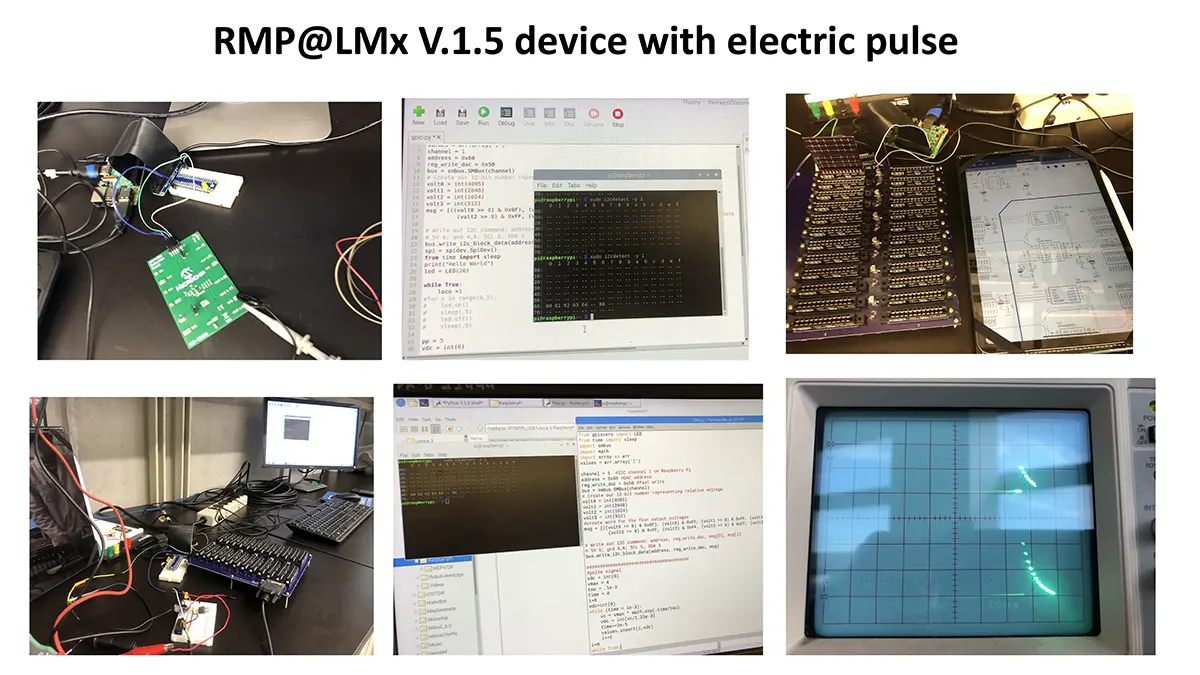
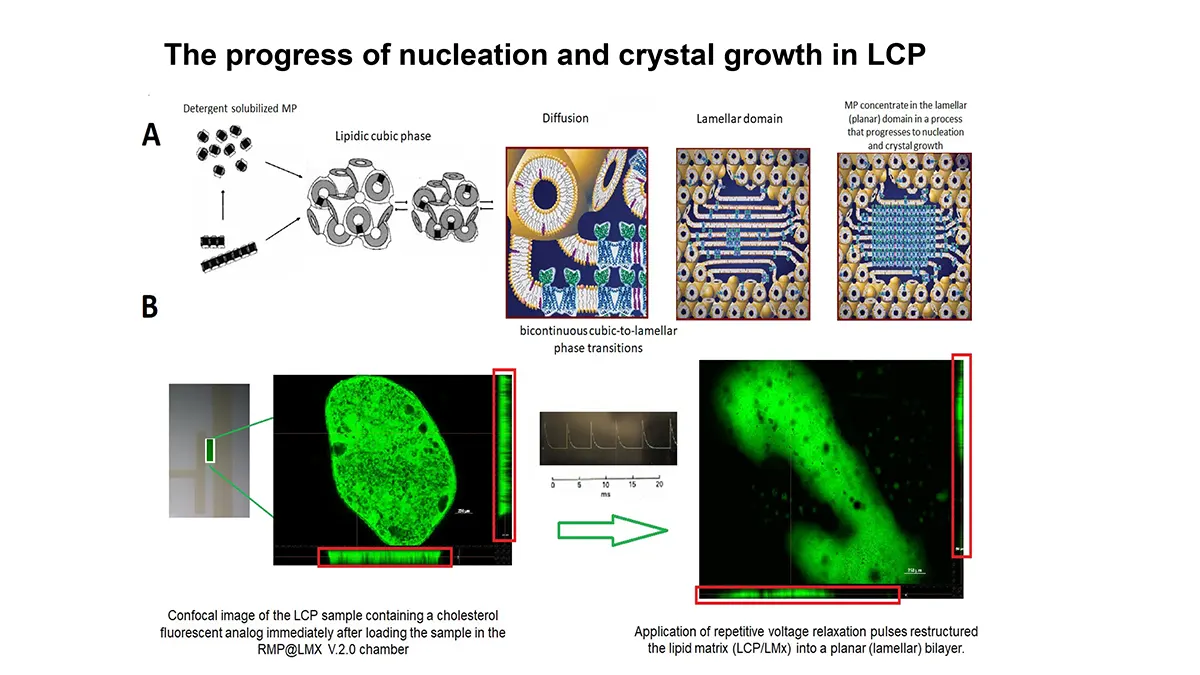
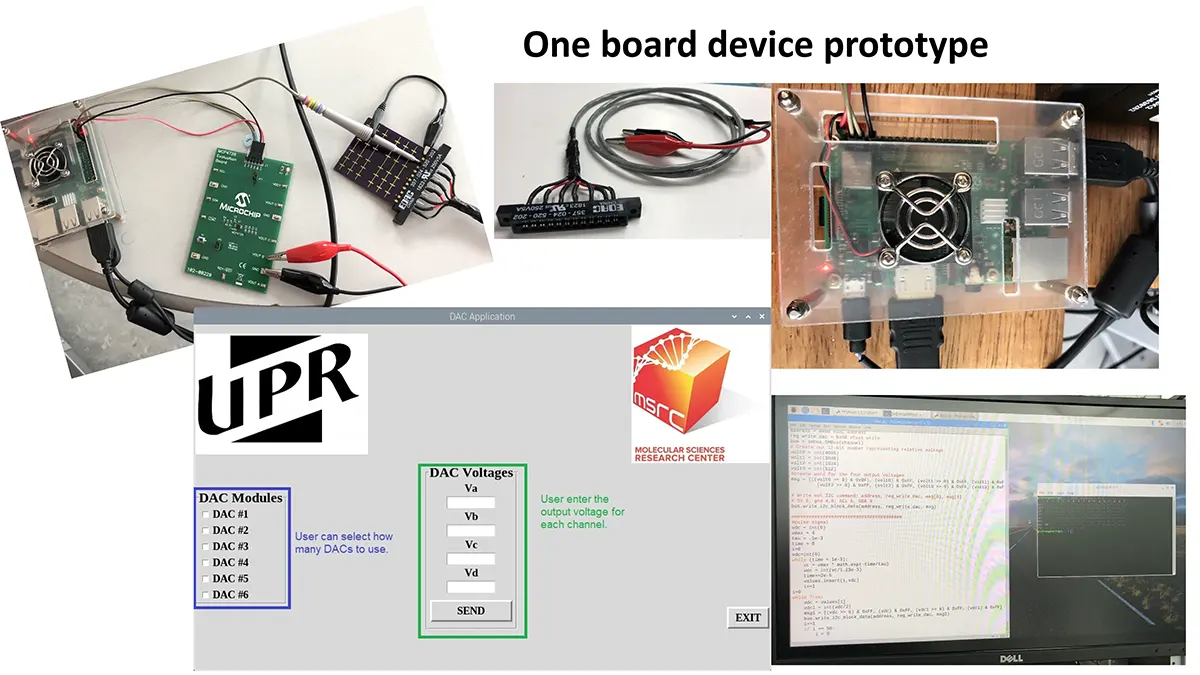
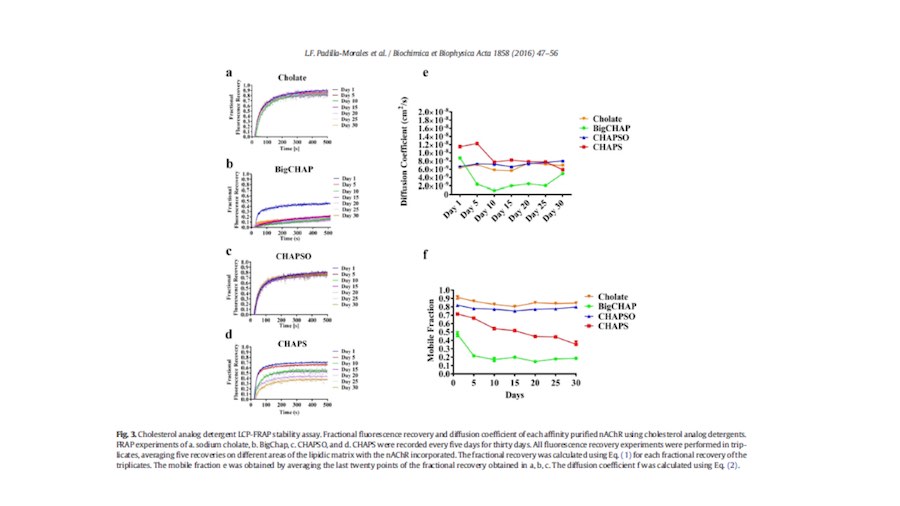
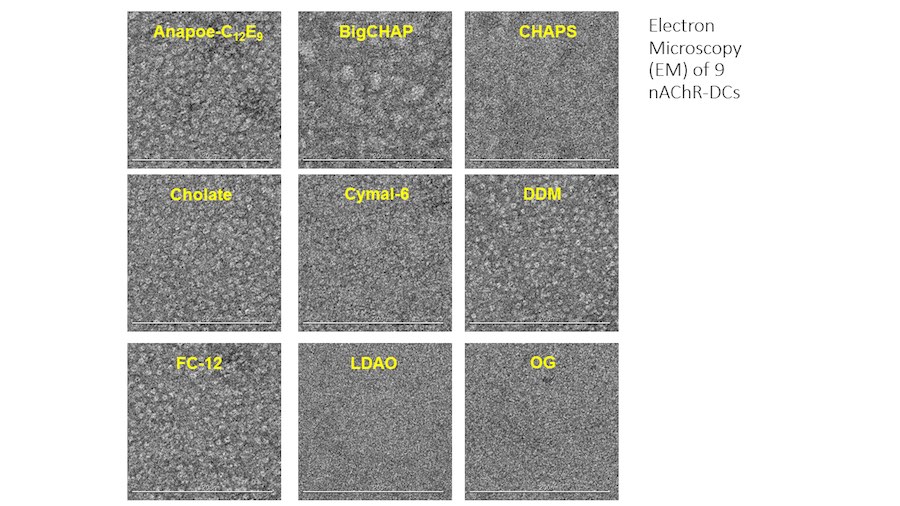
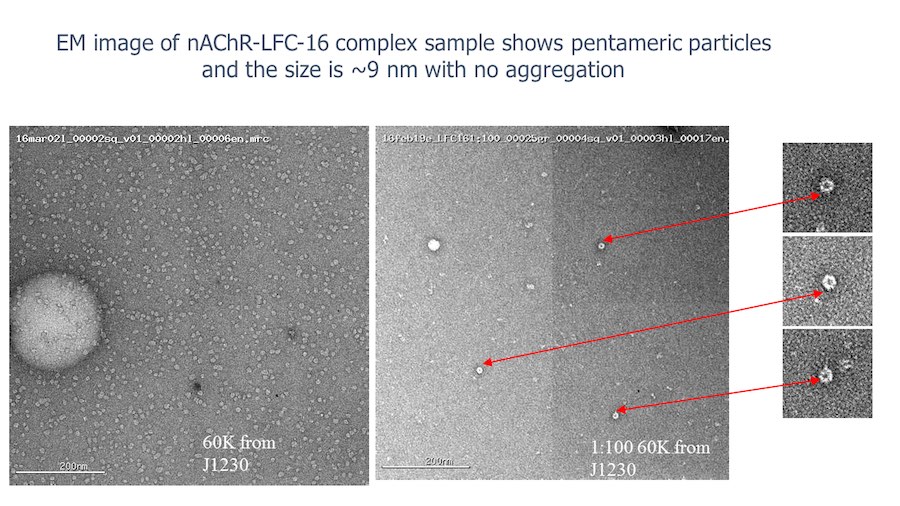
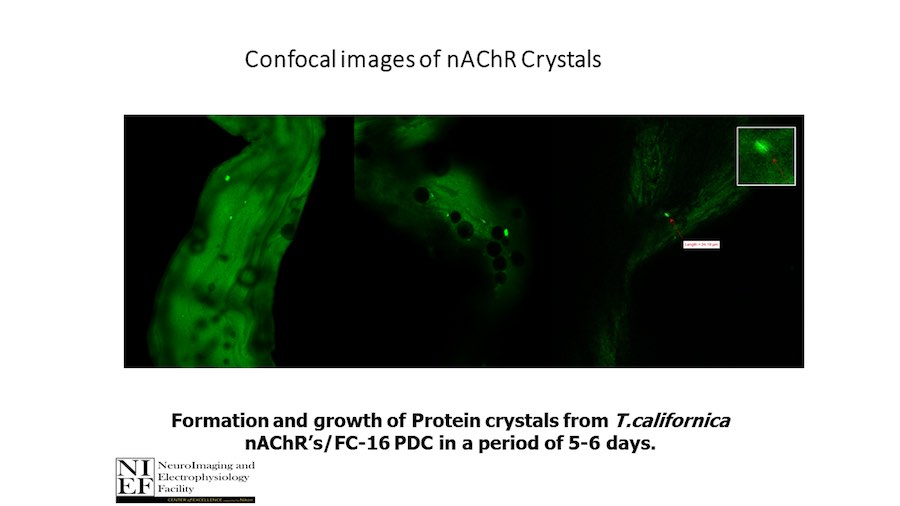
“After many years of intense electrophysiological recordings we prompted the idea of applying the patch clamp principle to the crystallization of membrane proteins. The biophysical basis for this idea emerged while performing single channel recordings in the cell-attached configuration at high acetylcholine concentrations. To rescue the Tc-nAChR from the desensitized state, we manipulated the resting membrane potential (RMP) in the patched membrane. The principle of arresting the conformational changes of a membrane protein using the RMP has never been conceived in a crystallization experiment. Our central hypothesis is that RMP can be used to restrict the conformation freedom of the membrane protein to facilitate crystallization. This hypothesis builds on strong preliminary data obtained using our first prototype of the RMP@LMx device (U.S. patent 10,155,221), engineered in our laboratory (www.nachrs.org) to crystallize the torpedo californica muscle-type nicotinic acetylcholine receptor (Tc-nAChR). The Tc-nAChR is a prime example of a multimeric membrane complex that is one of the most widely studied ion channels, but its high-resolution structure has remained elusive. We are now using the RMP@LMx V1.5 device to perform Resting Membrane Potential high-throughput crystallization screening (RMP-HTCS) of several nicotinic acetylcholine receptors. A high-resolution structure of the nAChR and its complexes with various nicotinic ligands is of crucial importance for the design of novel agents that target defined nervous system pathologies such as Alzheimer’s disease, schizophrenia, depression, attention deficit hyperactivity disorder, and tobacco addiction”. – JALD
The RMP@LMx U.S patent 10,155,221
The α7 nicotinic receptor is up-regulated in immune cells from HIV-seropositive women: Consequences to the cholinergic anti-inflammatory response
Antiretroviral therapy partially restores the immune system and markedly increases life expectancy of HIV infected patients. However, antiretroviral therapy does not restore full health. These patients suffer from poorly understood chronic inflammation that causes a number of AIDS and non-AIDS complications. We recently demonstrated that chronic inflammation in HIV+ patients may be due to the disruption of the cholinergic anti-inflammatory pathway (CAP) by the HIV envelope protein gp120IIIB. Our results demonstrate that HIV gp120IIIB induces an alpha7 acetylcholine receptor (α7) upregulation and a paradoxical pro-inflammatory phenotype in macrophages, as activation of the upregulated α7 is no longer capable of inhibiting the release of pro-inflammatory cytokines. Moreover our results demonstrate that HIV gp120IIIB disrupts the cholinergic-mediated anti-inflammatory response. These findings suggest that HIV tampering with a natural strategy to control inflammation could contribute to a crucial, unresolved problem of HIV infection: chronic inflammation. Our findings position the α7 neuronal nicotinic acetylcholine receptor as an attractive therapeutic target for the development of novel anti-inflammatory strategies to counteract the chronic inflammation suffered by HIV-infected patients.





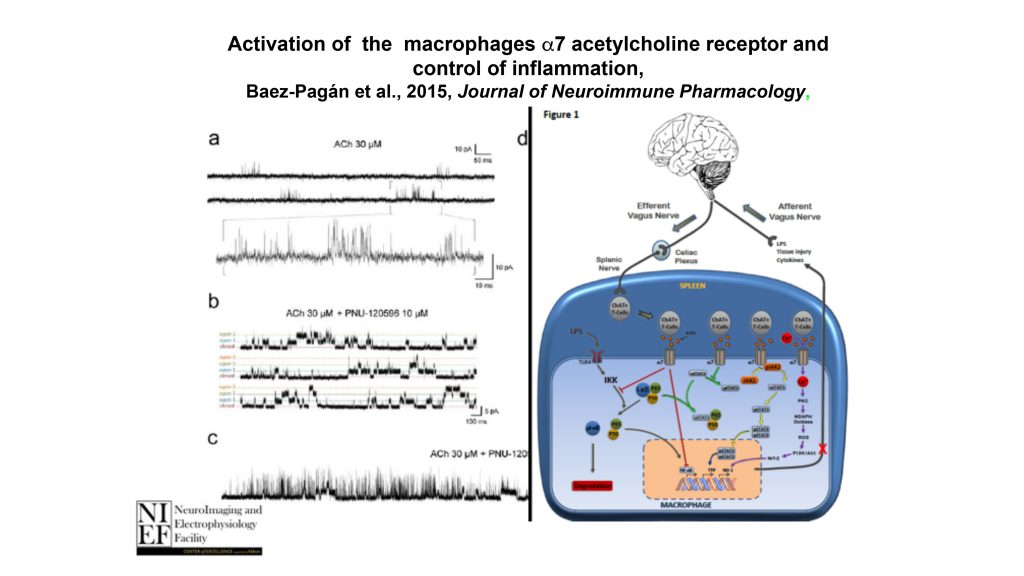
The α7 nicotinic acetylcholine receptor expressed in monocyte-derived macrophages retains its ion translocation activity
Uncontrolled inflammation may result in serious health problems that can be life-threatening. The α7 nicotinic acetylcholine receptor (α7-nAChR), a ligand-gated ion channel expressed in the nervous and immune systems, has an essential role in the control of inflammation. Activation of the macrophage α7-nAChR by acetylcholine, nicotine, or other agonists selectively inhibit the production of pro-inflammatory cytokines while leaving anti-inflammatory cytokines undisturbed. The neural control of this regulation pathway was recently discovered and it was named the cholinergic anti-inflammatory pathway (CAP). When afferent vagus nerve terminals are activated by cytokines or other pro-inflammatory stimuli, the message travels through the afferent vagus nerve, resulting in action potentials traveling down efferent vagus nerve fibers. This process eventually leads to macrophage α7-nAChR activation by acetylcholine and, consequently, inhibition of pro-inflammatory cytokine production. The mechanism through which activation of α7-nAChRs in macrophages regulates pro-inflammatory responses is subject of intense research, and important insights have thus been made. The results suggest that activation of the macrophage α7-nAChR controls inflammation by inhibiting NF-κB nuclear translocation and activating the JAK2/STAT3 pathway, among other suggested pathways. While the α7-nAChR is well characterized as a ligand-gated ion channel in neurons, whole-cell patch clamp experiments suggest that α7-nAChRs ion channel activity, defined as the translocation of ions across the membrane in response to ligands, is absent in leukocytes; therefore, ion channel activity is generally assumed to not be required for the operation of the CAP. We recently recorded single-channel currents as evidence that the α7-nAChR expressed in macrophages retains its ion translocation activity despite the absence of whole-cell currents. However, whether this ion-translocating activity is relevant for the proper operation of the CAP or other important physiological processes remains obscure. Presently, ongoing experiments in our laboratory are aimed to establish a potential mechanistic relationship between the α7-nAChR ion-translocating activity and the CAP.
Alpha7 nAChRs as a novel therapeutic target in the neuropathogenesis of HAND
The goal of this project is to characterize a novel mechanism that could be implicated in the pathogenesis of HIV-associated neurocognitive disorders (HAND). HAND is characterized by injury to specific brain areas, such as the cortex, hippocampus, and striatum. A key protein associated with the neurological complications of HIV, gp120, forms part of the viral envelope, and can be found in the cerebrospinal fluid (CSF) of infected individuals. Recently, we uncovered a novel pathway for gp120-induced neurotoxicity, in which activation of the CXCR4 receptor by gp120 leads to activation of the MAP kinase, increased EGR1 levels, and eventual up-regulation of the α7-nicotinic acetylcholine receptor (α7-nAChR) that triggers cell death through increased calcium influx. We confirmed the CXCR4-mediated α7-nAChR upregulation in a gp120-transgenic mice (gp120-tgm) brain, specifically in the striatum, a region severely affected in HIV+ patients. Our main hypothesis is that the α7-nAChR is a key mediator of the gp120-induced increase in calcium and cell death, and a potentially new therapeutic target for the treatment of HAND.
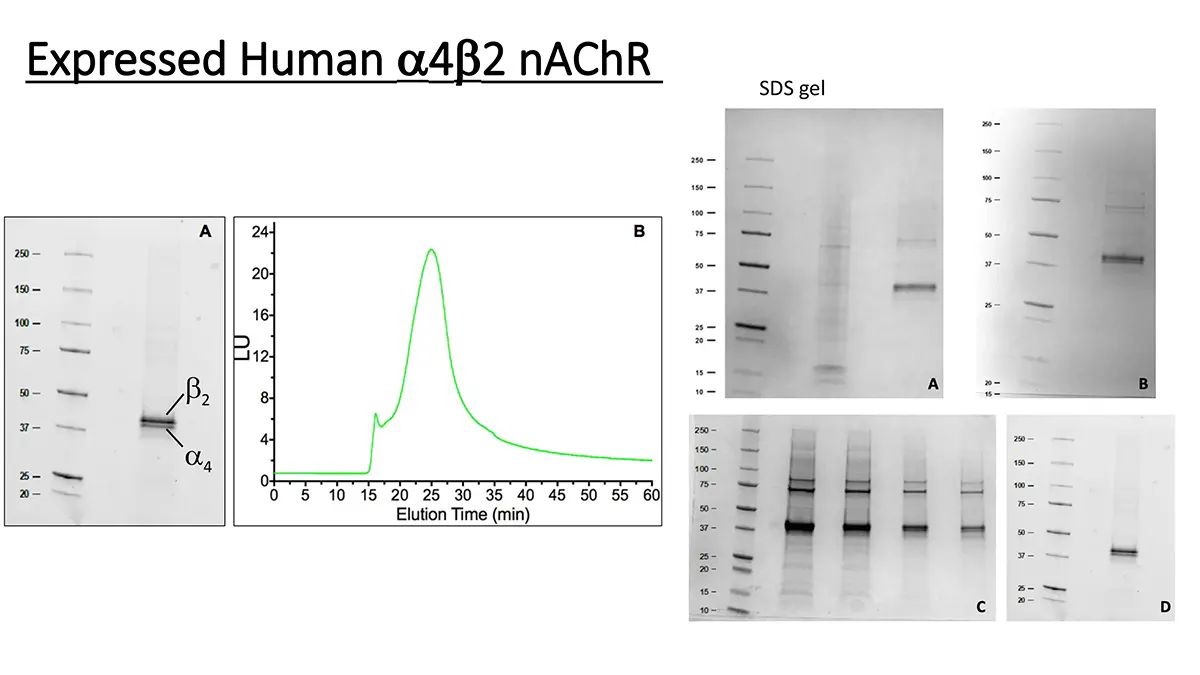
Engineering α4β2 nAChRs with reduced or increased nicotine sensitivity via selective disruption of consensus sites in the M3-M4 cytoplasmic loop of the α4 subunit
The α4β2 neuronal nicotinic acetylcholine receptor (nAChR) plays a crucial role in nicotine addiction. These receptors are known to desensitize and up-regulate after chronic nicotine exposure, but the mechanism remains unknown. Currently, the structure and functional role of the intracellular domains of the nAChR are obscure. To study the effect of subunit phosphorylation on α4β2 nAChR function and expression, eleven residues located in the M3-M4 cytoplasmic loop were mutated to alanine and aspartic acid. Two-electrode voltage clamp and 125I-labeled epibatidine binding assays were performed on Xenopus oocytes to assess agonist activation and receptor expression. When acetylcholine (ACh) was used as an agonist, a decrease in receptor activation was observed for the majority of the mutations. When nicotine was used as an agonist, four mutations exhibited a statistically significant hypersensitivity (S438D, S469A, Y576A, and S589A). Additionally, two mutations (S516D and T536A), which displayed normal activation with ACh, displayed remarkable reductions in their sensitivity to nicotine. Binding assays revealed a constitutive up-regulation in these two mutations with reduced nicotine sensitivity. These results suggest that consensus phosphorylation residues in the M3-M4 cytoplasmic loop of the α4 subunit play a crucial role in the regulation of the α4β2 nAChR agonist selectivity and functional expression. Furthermore, these results suggest that disruption of specific interactions at PKC putative consensus sites can render α4β2 nAChRs almost insensitive to nicotine without substantial effects on normal nAChR function. Therefore, these PKC consensus sites in the M3-M4 cytoplasmic loop of the α4 nAChR subunit could be a target for smoking cessation drugs.
Uncovering the molecular basis for statin-induced neuromuscular adverse drug reactions (ADR)
There are almost twenty million people in the U.S. alone that are treated with statin drugs for the treatment of hypercholesterolemia. Furthermore, many millions more are now having statin drugs recommended by their doctors. One of the main concerns with this treatment is that a large number of patients at risk of coronary artery disease (CAD) are forced to prematurely terminate statin therapy due to statin-associated drug reactions (statin ADRs). A significant percentage of patients under statin treatment (approximately 14-20%) develop minor muscle aches, myalgias, weakness, and a smaller number develop stronger, persistent muscle pain, myopathy and rhadomyolysis. Among those leaving treatment, how many are at serious risk of CAD? This is an important question for which there is no statistical data available. However, it is clear that this problem affects a significant group of patients every year in the USA and worldwide. Therefore, there is an overwhelming need to understand the mechanisms underlying the ADRs of statins in muscle; however, a search in the CRISP data-base clearly shows that there has not been a significant research effort to investigate this paramount and fundamental biomedical problem. We characterized a transgenic mouse model expressing a cholesterol-sensitive nicotinic acetylcholine receptor (nAChR) mutation that contains high levels of caveolin-1 (Cav-1) in the muscle endplate. The αC418W transgenic mouse was produced by a single nucleotide polymorphysim (SNP rs137852808 in the nAChR α-subunit gene CHRNA1 ) that induced a caveolin binding motif (CBM). The αC418W transgenic model displays muscle degeneration and weakness similar to the adverse effects manifested in statin treated patients. Most of the clinical, pathologic, and biochemical aspects of the statin ADRs related to muscle degeneration are fully replicated in the cholesterol-sensitive αC418W transgenic mouse. The objectives of this project are: (1) to increase the number of hypercholesterolemic patients able to continue on statin treatment by characterizing the etiology of statin ADRs that are particularly intrinsic to the neuromuscular junction (NMJ), (2) assess therapeutic venues to ensure a safer and effective treatment and (3) develop predictive tools to assess the risk of developing statin ADRs in the general population.