Cholesterol regulation of acetylcholine channel function
The role of membrane cholesterol in the function of the nicotinic acetylcholine receptor (nAChR) has been studied by many groups (Guzman, G., et. al., 2002). Nevertheless, the role of lipid-protein interactions and in particular, the role lipid-exposed nAChR residues in the modulation exerted by cholesterol in the ion-channel function has only recently been studied.
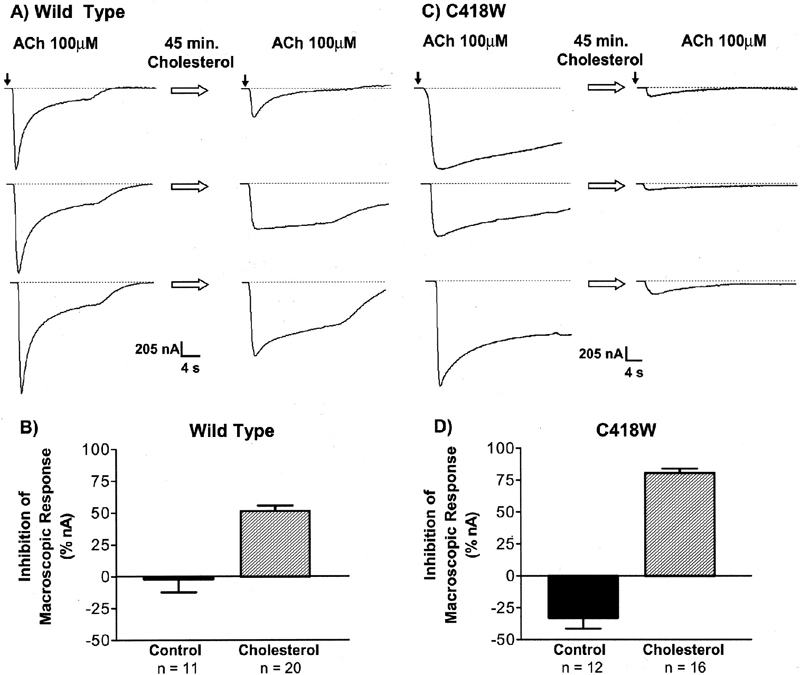
In the year 2001, our laboratory published a paper on the effects of membrane cholesterol in the function of the Torpedo californica nAChR (Santiago, et. al., 2001). Using Xenopus laevis oocytes as expression system, the consequences in the function of the Torpedo californica WT nAChR as well as the novel lipid-exposed αC418W mutant was studied. Methyl-β-cyclodextrin (MβCD) incubations were employed to remove cholesterol from the oocytes plasmalemma whereas cholesterol-enriched dioleoylphosphatidylcholine (DOPC) liposomes were used to increase the membrane cholesterol concentration. MβCD incubations, which efficiently remove cholesterol from the plasmalemma, resulted in a remarkable increase in the αC418W macroscopic response compared to the WT which displayed only a modest increase (Figure 1). Furthermore, cholesterol enrichment of the Xenopus laevis oocytes resulted in considerable decreases in macroscopic response in the αC418W when compared to the WT (Figure 2).
In essence, these studies highlighted the important role of lipid-protein interactions in potentiating the effect membrane cholesterol has on the nAChR function. Nevertheless, while several hypothesis were raised in Santiango, et. al., 2001 to explain these observations, the mechanism by which the αC418W was more sensitive to modifications in the concentration of membrane cholesterol remained to be elucidated.
To gain insight into the mechanism by which cholesterol depletion of the oocyte’s plasmalemma resulted in an outstanding increase in the macroscopic response of the αC418W nAChR, several studies were undertaken. In brief, to rule out the possibility that cholesterol depletion may be having an effect on the αC418W nAChR single-channel kinetics or conductance, Patch clamp experiments in the cell-attached configuration were performed. As reported by Baez-Pagan, et. al., 2008, neither single-channel kinetics nor conductance of the αC418W nAChR were modified upon cholesterol depletion (Figure 3). These results raised the hypothesis that cholesterol depletions could be, alternatively, increasing the pool of functional receptors (activatable pool). In an attempt to reveal a potential source of additional functional receptors, confocal microscopy experiments were performed. These experiments revealed clusters of receptors located at focal planes beyond the membrane surface (Figure 4.A). When comparing the clusters formed by the WT vs. αC418W nAChR, it was determined that the clusters formed by the lipid-exposed mutation were both bigger and more densely distributed. Furthermore, the clusters formed by the αC418W nAChR were more sensitive to cholesterol levels as cholesterol depletion significantly decreased the amount of clusters/μm2 whereas not in the WT (Figure 4.B-E). Careful inspection of the sequence of amino acids in the fourth transmembrane domain (M4) revealed that substitution of cysteine by tryptophan at position 418 introduced a caveolin binding motif (CBM). Co-fractionation and co-immunoprecipitation of the αC418W nAChR and caveolin 1 (Cav 1) further implicated caveolin-positive domains as having an important role in the regulation of the αC418W nAChR by cholesterol. For more details see Baez-Pagan, et. al., 2008 in the Publications section.
In the course of this investigation, the αC418W nAChR was shown to be the caused of a Slow-Channel Congenital Myasthenic Syndrome (SC-CMS) in humans (Shen X-M, et. al., 2006) thus highlighting the relevance of the lipid-protein interface in the regulation of the nAChR function and supporting the notion of altered lipid-protein interactions as potential cause for disease.
A natural evolution of these studies was to probe these effects in vivo. For such studies, we developed a transgenic mouse model in collaboration with Dr. Christopher Gomez from the University of Chicago. Using the αC418W transgenic model, we study the effects of a daily dose of Lipitor® (atorvastatin calcium) in the nAChRs present in diaphragm muscle endplates, lateral diffusion of endplate receptors, miniature endplate potentials, compound muscle action potentials and mice locomotor activity as well as the role of caveolin isoforms and cation overloading in endplate regulation. The significance of these results rests upon the potential implications of the altered functional and nonfunctional pools of nAChRs in humans using cholesterol-lowering drugs as a potential cause for the most serious side-effects. Furthermore, the results from these experiments raise the possibility that statins could uncover genetic defects in humans.
Understanding the molecular basis for the side-effects of statins is critical to prevent the collateral contraindications and also to improve therapeutic intervention in hypercholesterolemic patients. Indeed, high LDL levels place individuals at risk of suffering from coronary heart diseases. Nevertheless, while statin treatment has been successfully used to decrease serum LDL levels in hypercholesterolemic patients, a significant number of those treated suffer from unexplained painfull collateral effects impeding continued medication. Therefore, if more hypercholesterolemic patients are to benefit from low LDL levels, the underlying mechanisms behind these collateral effects must be thoroughly studied.